Bryant11
Newbie

My vehicle always intermits. Does it mean the starter Relay get bad? What are the symptoms that the Starter Relay is going bad?
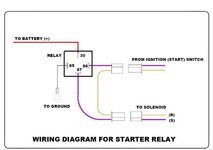
Also, it is interesting to know the function of starter relay. Share with you.
Oh, if you want to know more about its knowledge, you can read this article. But I still don't know why my car always intermit! Who can tell me?
Also, it is interesting to know the function of starter relay. Share with you.
--- Updated ---
Oh, if you want to know more about its knowledge, you can read this article. But I still don't know why my car always intermit! Who can tell me?